By Brian Stallard, Media Relations Manager, AAMI
When someone talks about the “frontlines” of healthcare, you likely envision harried doctors racing from room to room, seeing as many patients as they can while resilient nurses work long hours to deliver care. Appreciation for healthcare’s frontline workers, especially during a global health crisis like the COVID-19 pandemic, should never be understated. There is, however, another group of tireless frontline workers that you may not know exist!
“When you hear about ventilators and other important medical equipment that’s being used to treat patients, healthcare technology management, or HTM, professionals are the folks that keep it all running. They’re on the front lines with the rest of the hospital staff,” explained Robert Burroughs, senior vice president of education for the Association for the Advancement of Medical Instrumentation (AAMI).
During the COVID-19 pandemic, it was HTM professionals who burned the midnight oil to quickly set up desperately needed ventilators, prepare (and sometimes 3D-print) new personal protection equipment (PPE) for caregivers, and maintain extensively used emergency devices.
“A truly heartfelt thank-you to the HTM professionals on the front lines of the COVID-19 pandemic. Your hard work, courage, diligence, and commitment to healthcare institutions and the patients they serve has been nothing short of heroic,” said Danielle McGeary, AAMI’s vice president of HTM, during the Association’s recent AAMI eXchange – a showcase, learning, and networking event aimed at HTM professionals.
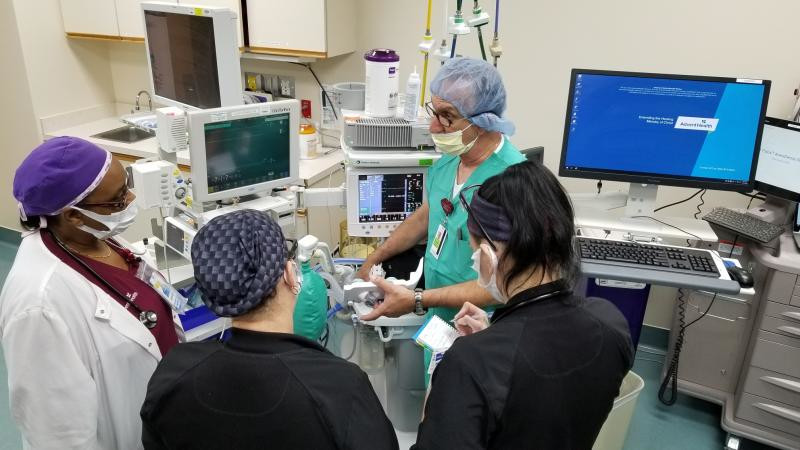
Crothall anesthesia service engineer Carlo Bracci, Jr., teaches the clinical anesthesia teams at each facility how to convert the units into ventilators.
Building a Frontline Pipeline
There’s little doubt that these are essential workers. According to the U.S. Bureau of Labor Statistics, there are an estimated 53,900 positions for medical equipment repairers, also known as biomedical equipment technicians (BMETs), in the United States alone, which fall under the HTM umbrella. In the next 10 years, employment of BMETs is projected to grow five percent, faster than the average for all U.S. occupations.
And yet, many HTM departments faced this public health crisis with a shortage of personnel. AAMI’s 2020 survey of 71 healthcare organizations and a sampling of more than 7,000 HTM professionals revealed that HTM departments across the U.S. are 8.5% understaffed (open vacancies) on average. Explaining for vacancies, one-third of those organizations reported that it takes two to four months to fill a position while another 30% reported that it takes longer than four months to fill a position.
What’s more, nearly half the HTM staff currently employed will soon to retire, and a worrying percentage of those retirements will occur in key management positions. Among respondents holding managerial positions, nearly 6 in 10 reported being 50 or order, with more than 15 percent over the age of 60.
Exacerbating the problem, “U.S. colleges are being forced to drop their BMET programs due to budgetary constraints,” said McGeary. “This only serves to widen a training gap between the county’s most senior and soon to be retiring BMETs and the next generation of HTM professionals.”
Fortunately, one of AAMI’s core strategic initiatives is to help increase the HTM personnel pipeline.
AAMI’s new BMET Apprenticeship program approved by the U.S. Department of Labor matches prospective apprentices with organizations nationwide. Participating organizations are offered state tax credits for taking on apprentices. The National Apprenticeship Act of 2021, recently passed by the U.S. House of Representatives, may also provide support as soon as early 2022.
Healthcare’s Second Front
Making sure well-trained professionals are ready to manage sophisticated healthcare devices is only half the battle. During the worst of the COVID-19 pandemic, the professionals involved in the development and regulation of medical devices also worked tirelessly to ensure hospitals and treatment centers were equipped to take on a global health crisis.
For more than 50 years, AAMI has been at the forefront in developing voluntary consensus standards and technical information reports for the medical device space. These documents, developed through the coordination of more than 2,500 volunteers from around the world, are used to ensure the safe and effective production, distribution, and use of health technology.
Standards also aid the regulation of these essential products, as the documents serve as state-of-the-art guidelines for regulatory compliance. Representatives from the U.S. Food and Drug Administration (FDA) participate in many of AAMI’s more than 150 standard committees and working groups, while representatives from AAMI Standards join crucial discussions with other international standards developers in global committees.
When the COVID-19 pandemic created an unprecedent need for the fast development of new devices and maintenance practices under FDA Emergency Use Authorization (EUA), companies and amateur engineers alike hastened to invent inexpensive solutions for COVID-19 patient care.
Unfortunately, when hospitals are filling, there’s no time to develop new standards for emergency-use devices. Nor were some well-meaning companies aware of the existing standards for medical devices.
“Early in the pandemic, there was uncertainty,” said Sandy Weininger, senior engineer at the FDA’s Center for Devices and Radiological Health and member of AAMI’s Anesthetic and Respiratory Equipment (AR) committee. “You had this perfect storm of scarcity of parts, testers who aren’t in labs, and device constructors trying to innovate in spaces they’re not entirely familiar with.”
“We weren’t really sure at that time what problems were going to surface or how clinical practice might have to change abruptly,” added Julian Goldman, anesthesiologist, medical director of biomedical engineering, director of the Medical Device Interoperability & Cybersecurity Program at the Mass General Brigham Health System and member of the AR committee. “Many of us are used to working together. And so, we asked AAMI to leverage existing relationships and help us convene meetings very quickly.”
The resulting team was dubbed the AAMI COVID-19 Response Team. With Goldman and Weininger as co-chairs, the committee of well over 100 experts has addressed some of the most pressing device-related concerns for caregivers and regulators during the COVID-19 pandemic. This included emergency design and user guidelines for resuscitators, BiPAP, ventilatory helmets, and even guidance for introducing remote-control capabilities for devices in high-risk patient rooms.
In all, the team rapidly produced 10 emergency guidance documents which were made immediately and freely available to the public. These documents are a first-of-their-kind standards resources, called consensus reports (CRs).
“CRs are intended to provide a very concise and practical guidance on a really narrow topic,” added Amanda Benedict, vice president of standards, AAMI. “The emergency response CRs are useful for responding to areas where there’s an immediate need for guidance. So, they’re perfect for a public health emergency.”

CURE Biomedical employees in Sunnyvale, CA, get hundreds of vital ventilators tested and ready for use.
This Isn’t the End
Naturally, once the emergency ends, the FDA and other regulating bodies across the world will be lifting their EUAs. Case in point, the FDA has already revoked EUAs for disposable respirators not approved by National Institute of Occupational Safety and Health (NIOSH), as well as some decontamination equipment. Once an EUA is lifted, devices that have not gone through the proper pre-COVID approval channels will no longer be allowed to be used or sold.
And yet, those who followed the emergency CRs may have an edge as they consult established standards and seek regulatory approval. That’s because, in following the ten CRs, manufacturers and caregivers have already been exposed to a refined sampling of what is the state-of-the-art for the safe (and potentially regulator compliant) development and use of medical devices. To write these CRs, “we’ve leveraged existing device standards while narrowing the scope, slicing up the apple and only taking the immediately useful parts,” Weininger explained.
COVID Response Team member Bob Kopotic, senior manager of clinical and medical affairs for critical care at Edwards Lifesciences, added that the communal effort put into creating these CRs also created a culture of information sharing rarely seen in the medical device field. “It was tremendous to see the number of otherwise competitor ventilator companies come together in a very collegial way, “he said.
Contributing manufacturers “realized that they are in some way supporting use of devices where otherwise their own device would be in place,” and yet, it didn’t seem to matter. “I see these people passionate about patients, and that, I think, really was the drive.”
AT A GLANCE
Association for the Advancement of Medical Instrumentation (AAMI)
What: A non-profit organization for the development, management, and use of safe and effective health technology
Where: Arlington, Virginia
Website: www.aami.org


